Regulation and
standards
Regulations and standards relating to laboratory gloves and cleanroom gloves
Laboratory gloves and cleanroom gloves must meet specific Regulations and standards.
In Europe, they should be PPE first (Personal Protective Equipment), top protect the wearer according to Regulation (EU) 2016/425. But they can also be MD (Medical Device) to protect the patient according to Regulation (EU) 2017/745.
They also meet specific standards depending on the risk they are intended to cover. There are many norms relative to disposable gloves depending on the use for which they are intended to. We introduce you to the Regulations and standards relating to Lab and Cleanroom gloves to help you select the right glove according to the hazards encountered.
Difference between Regulations and Standards:
Regulations are binding legislative acts. Regulations have legal force throughout every Member State of the European Union (EU).
A standard is a document that provides requirements, specifications, to ensure that materials, products, processes, and services are fit for their purpose. They proceed from the main stakeholders and must be ratified by an International or European Standardization organization (e.g., ISO, CEN …). Furthermore, standards are voluntary: there is no legal obligation to apply them. However, laws and regulations may refer to Standards.
The Regulation (EU) 2016/425 lays down the general requirements for the design and manufacture of Personal Protective Equipment (PPE).
The Regulation (EU) 2016/425 have superseded the Directive 89/686/EEC.
The previous Directive placed responsibility on the manufacturer while the Regulation (EU) 2016/425 assigns responsibility on the whole supply chain. This means everybody is involved: manufacturers, importers but also distributors who must also ensure themselves that the PPE meet all the requirements according to this Regulation without only relying on the manufacturer.
PPE gloves are categorized as Simple Design (often referred to as Category I) or Complex Design (Category III). Intermediate design (Category II) gloves are those gloves that do not fall into either Complex Design or Simple Design categories.
Simple Design gloves are defined as those gloves that protect the wearer from cleaning materials of weak action and easily reversible effects. Considering that it clearly appears that Simple Design gloves have a limited protection role in the workplace where protection from chemicals and micro-organisms is sought.
Complex Design, in contrast, covers the highest level of risk, otherwise defined as irreversible and mortal risk. Disposable gloves in this category (PPE Cat III) are typically those gloves that provide protection against chemical and microorganisms. For these gloves the following key normative references may apply:
- ISO 21420:2020 (general requirements for gloves)
- ISO 374-1:2016+A1:2018 (terminology and performance requirements for chemical risks)
- ISO 374-5:2016 (terminology and performance requirements for micro-organisms risks).
Complex Design brings the need, whether for gloves CE or UKCA marked, for regular auditing by an external organization body, called a Notified Body. The presence of the Notified Body is clear, as under the CE or UKCA mark will appear four digits (e.g., 0598 = SGS, 0493 = Centexbel, 0123 = TÜV etc). The Notified Body validates the quality assurance system used by the manufacturer.
In addition, disposable gloves that have been registered as Complex Design will typically display two or three pictograms identifying the relevant standards to which they have been tested.

PPE gloves' general EU requirements
Marking attesting European compliance requirements

Marking attesting UK compliance requirements
The Regulation (EU) 2017/745 regarding Medical Devices (MDR) have superseded the Directive 93/42/EEC.
This new Regulation places a stronger emphasis on transparency, patient safety with a stricter pre-market control and a reinforced post market vigilance via EUDAMED registration, UDI-DI number for traceability, 5 years validity for the certificates, shared responsibilities by all the stakeholders…
The primary concern for MDR is protecting the patient.
Underneath the CE mark, a reference to the standard EN455 “Medical gloves for single use” may sometimes features providing easy identification. Typically, non-sterile gloves that are registered according to the MDR are labelled on the packaging as “Exam Gloves” or “Medical Examination Gloves”, highlighting their role in patient care. It should be noted that these gloves are considered class 1 medical devices and as such undergo a self-certification process that is conducted directly by the manufacturer. Unlike sterile exam gloves or surgical gloves, there is no independent validation of the test data by an external organization.

European Regulatory framework for medical devices
The standard ISO 21420:2020 replaced the EN 420:2003+A1:2009. This standard is now a worldwide standard.
The ISO 21420:2020 specifies the general requirements and test methods applicable to all protective gloves for :
- Glove design
- Construction
- Resistance of glove materials to water penetration
- Innocuousness
- Comfort
- Efficiency
- Marking
- Information supplied by the manufacturer
Understanding ISO 21420:2020 requirements and obligations
Wearing the appropriate protective glove when working with chemicals and/or micro-organisms is of utmost importance otherwise it may result in serious injuries and sometimes irreversible. The ISO 374 is related to protective gloves against chemicals and micro-organisms. This norm is divided in several parts under 2 main topics:
CHEMICAL PROTECTIVE GLOVES
Mains standards related to the chemical risk are:
- Norm ISO 374-1:2016+A1:2018 which refers to the “Terminology and performance requirements for chemical risks”.
- Norm EN 16523-1:2015+A1:2018 which refers to “Permeation by liquid chemical under conditions of continuous contact”.
- Norm ISO 374-2:2019 which refers to “Determination of resistance to penetration”.
- Norm ISO 374-4:2019 which refers to “Determination of resistance to degradation by chemicals”.
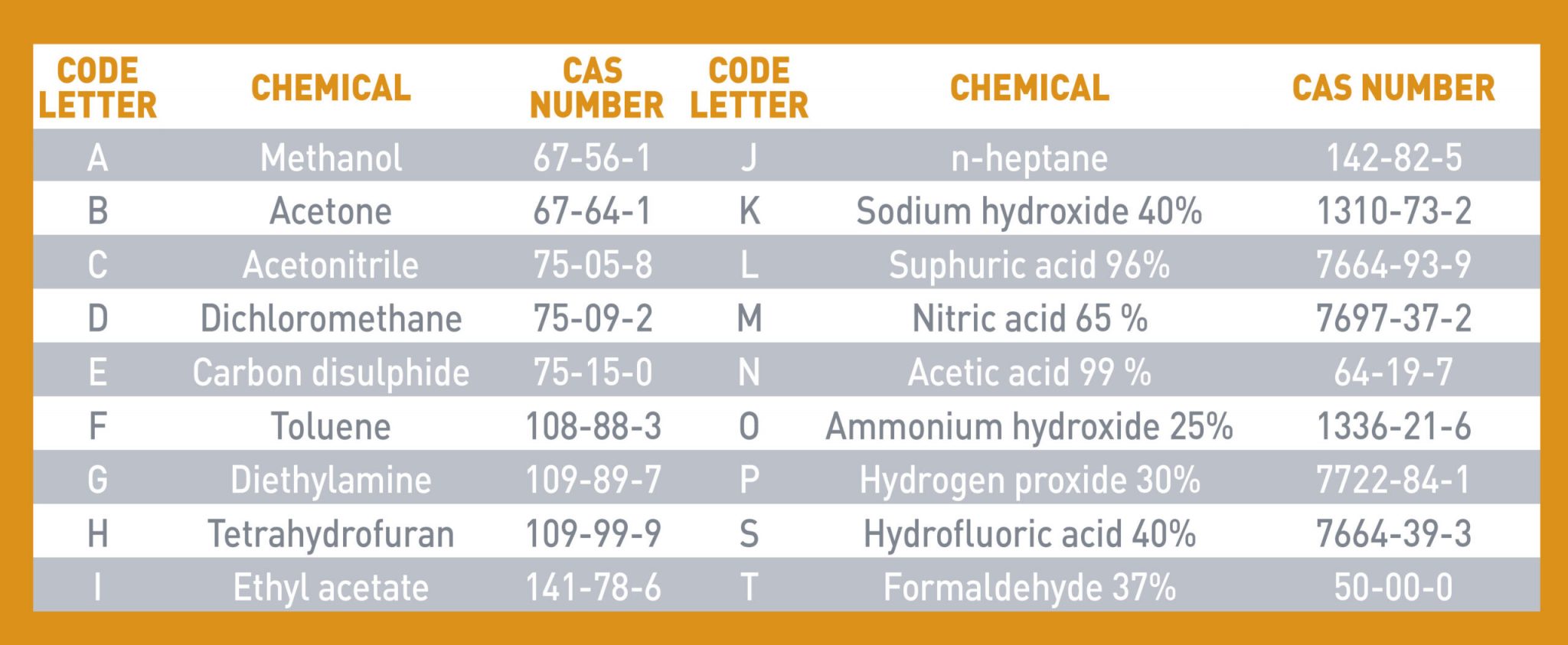
Gloves are classified as Type A, B or C depending on their performance level when tested against one or more chemicals (see list in the table below) and degradation expressed in terms of mean average (% change in puncture resistance before and after chemical exposure):
- TYPE A: Minimum performance level required is Level 2 meaning the gloves must resist at least 30 minutes against 6 of the 18 chemicals listed in ISO 374-1:2016+A1:2018. The chemicals tested shall be identified by their code letter under the chemical hazard pictogram.
- TYPE B: Minimum performance level required is Level 2 meaning the gloves must resist at least 30 minutes against 3 of the 18 chemicals listed in ISO 374-1:2016+A1:2018. The chemicals tested shall be identified by their code letter under the chemical hazard pictogram.
- TYPE C: Minimum performance level required is Level 1 meaning the gloves must resist at least 10 minutes against 1 of the 18 chemicals listed in ISO 374-1:2016+A1:2018. The chemical tested shall be identified by its code letter under the chemical hazard pictogram.
Below 18 test chemicals listed in the ISO 374-1:2016+A1:2018:



BIOLOGICAL PROTECTIVE GLOVES
Mains standards related to the biological risks are:
- Norm ISO 374-5:2016 which refers to “Terminology and performance requirements for micro-organisms risks”.
- Norm ISO 374-2:2019 which refers to “Determination of resistance to penetration”.
- Norm ISO 16604:2004 Procedure B which refers to “Clothing for protection against contact with blood and body fluids – Determination of resistance of protective clothing materials to penetration by blood-borne pathogens – Test method using Phi-X174 bacteriophage”.
ISO 374-2 :2019 remains the main test for assessing resistance to penetration by micro-organisms. The performance is measured in terms of AQL (Acceptable Quality Level).
The lower the AQL, the better the level of protection against micro-organisms penetration:
AQL<4 or Level 1, AQL<1.5 or Level 2, AQL <0.65 (including AQL 0.25) or Level 3. Level 3 being the highest performance level.
For protective gloves against bacteria and fungi, the biohazard pictogram is applied.
For protection against bacteria, fungi, and virus, the biohazard pictogram is accompanied with the term “VIRUS “underneath.
To fulfil this requirement, the glove must be tested according to ISO 374-2:2019 for bacteria and fungi and in addition tested according to ISO 16604: 2004 (Method B) using the bacteriophage penetration test.


Reasons for using gloves in cleanrooms and controlled environments are:
- Worker safety
- Cleanliness of the process and product
In a cleanroom or in controlled environments, the number of particles, germs and bacteria in the air must remain as low as possible.
This number depends on many factors such as air flow, air humidity, pressure, or ambient temperature but also human movements, equipment’s and consumables used in the cleanroom. The IEST RP-CC005.4 standard describes procedures for testing and evaluating gloves in terms of physical attributes and cleanliness. Tests are provided for determining cleanliness (particle release, extractables, Non-Volatile Residue (NVR), detections of chemicals such as Silicone or DOP via infrared spectroscopy (FTIR)…), physical and chemical integrity, and other relevant properties.
For a glove to be used in a medical environment, it is essential that it meets the requirements of the 4 norms constituting the EN 455 series relating to “Medical gloves for single use”.
The 4 norms are:
- EN 455-1:2020: Requirement and testing for freedom from holes.
- EN 455-2:2015: Requirement and testing for physical properties.
- Medical gloves dimensions
- Surgical gloves and exam gloves sizes
- Procedure gloves and surgical gloves strength
- EN 455-3:2015: Requirement and testing for biological evaluation.
- Chemicals
- Endotoxins (sterile gloves only)
- Powder
- Natural Rubber Latex Proteins
- EN 455-4:2009: Requirement and testing for shelf-life determination.
- Shelf life and resistance to degradation
- Sterile barrier integrity
- Storage conditions
This standard refers to “Protective gloves against mechanical risks”.
It covers mechanical risks such as abrasion, blade cut, tear, puncture and if applicable impact. A pictogram identifying a glove offering protection against mechanical risks will have underneath up to four numbers and where appropriate up to two letters.
This pictogram can only be displayed if the glove achieves a performance level rating of one in at least one of the four specific tests.

This standard refers to “Protective gloves against ionizing radiation and radioactive contamination”.
It is divided into two parts:
- Protective gloves against radioactive contamination: Such gloves should comply with the ISO 21420 Standard, they should achieve an AQL 1.5 according to ISO 374-2:2019 and at least they must feature a level of performance of 1 for one of the five mechanical risks tests according to EN 388:2016+A1:2018 (Special note: dexterity could be the most important parameter, in this context no level of mechanical protection is required but then it should be written in the user notice of the gloves: “This gloves does not protect against mechanical risks.”).
- Protective gloves against ionizing radiation: The requirements are the same as described above. In addition, the glove shall demonstrate an efficiency to absorb radiation (lead equivalent thickness). The minimum uniformity of distribution of protective material (lead) is 0.05 mm. None of the SHIELD Scientific disposable gloves meets this part of the norm.
The EN421:2010 also describes the performance requirements regarding gloves for containments enclosures.
ESD marked garments must dissipate electrostatic charges to avoid the risk of sparks that could cause a fire or even an explosion in certain risk areas. Antistatic clothing is frequently used in ATEX zones (for French terms “ATmosphère EXplosible” meaning explosive atmosphere).
The norm EN 1149 consists of several parts under the general title “Protective clothing. Electrostatic properties”:
- Part 1 (EN 1149-1:2006): Test method for measurement of surface resistivity.
- Part 2 (EN 1149-2:1997): Test method for measurement of the electrical resistance through a material (vertical resistance).
- Part 3 (EN 1149-3:2004): Test methods for measurement of charge decay.
- Part 4 (norm under development): Garment test method.
- Part 5 (EN 1149-5:2019): Material performance and design requirements.
But note that at present, there is no generic norm to define the ESD performance of a disposable glove and that gloves are excluded from the norm EN 1149-5:2019.
Learn more about ESD disposable gloves by reading our dedicated article. Learn more
The norm EN 16350:2014 for Protective gloves – Electrostatic properties, provides additional requirements for protective gloves that are worn in ATEX zones. EN 16350:2014 standard goes beyond the EN 1149-2:1997 standard. For example, while using the same test method, the EN 16350:2014 standard sets the performance requirements for the vertical resistance properties of a glove: Rv < 1.0 × 108 Ω.
ASTM D6978-05 (2019) is a Standard Practice for Assessment of Resistance of Medical Gloves to Permeation by Chemotherapy Drugs.
The ASTM D6978-05 (2019) norm has been specifically developed for operators exposed to cytotoxic agents. Furthermore, it is hundred times more sensitive than the European equivalent test (EN 16523-1:2015 supersedes EN 374-3:2003) what is a criterion to be taken into account for selecting gloves when handling cytotoxic drugs.
You want to ensure that you’re using the right glove for protecting yourself and the product?
Have a look at our quick comparison of these two standardsLearn more
What is and what is not true: consult our checklist to help you selecting the highest level of protectionLearn more
There are many other Regulations and standards which apply depending on the country/continent or the framework requested.
Here are some you will encounter:
- NSI/AAMI/EN ISO 1137-2:2015 – Gamma Sterilization Dose Auditing
- ASTM D257-07 – Standard Test Methods for DC Resistance or Conductance of Insulating Materials
- ASTM D257-36 – Standard Test Methods for DC Resistance or Conductance of Insulating Materials
- ASTM D3578-05 – Standard Specification for Rubber Examination Gloves
- ASTM D3767-03 (2020) – Standard Practice for Rubber—Measurement of Dimensions
- ASTM D412-06ae2 – Standard Test Methods for Vulcanized Rubber and Thermoplastic Elastomers—Tension
- ASTM D5712-15 (2020) – Standard Test Method for Analysis of Aqueous Extractable Protein in Natural Rubber and its products using the Modified Lowry Method
- ASTM D6124-06 – Standard Test Method for Residual Powder on Medical Gloves
- ASTM D6319-10 – Standard Specification for Nitrile Examination Gloves for Medical Application
- ASTM F1671-97b – Standard Test Method for Resistance of Materials Used in Protective Clothing to Penetration by Blood-Borne Pathogens Using Phi-X174 Bacteriophage Penetration as a Test System
- ASTM F720-81 – Standard Practice for Testing Guinea Pigs for Contact Allergens: Guinea Pig Maximization Test
- ASTM D573-04 (2019) – Standard Test Method for Rubber—Deterioration in an Air Oven
- Directive 72/2002/EC – relating to plastic materials and articles intended to come into contact with foodstuffs
- EC 1935/2004 – Directive on materials and articles intended to come into contact with food
- FDA 21CFR177.2600 – FDA – Food for Human Consumption – Indirect Food Additives: Polymers – Rubber article intended for repeated use
- ISO 21171:2006 – Medical gloves – Determination of removable surface powder
- ISO 9001:2015 – Quality management systems — Requirements
- ISO 13485:2016 – Medical devices – Quality management systems — Requirements for regulatory purposes
- ISO 14001:2015 – Environmental management systems
European regulations, national or international standards... Understanding the Regulations and Standards relating to Laboratory and Cleanroom Gloves is not easy! However, you must master and take them into account when selecting a lab glove or a cleanroom glove. The SHIELD Scientific team is at your disposal to accompany you and enlighten you on all these subjects. Contact us!