CLEANROOM GLOVES’ PERFORMANCE FOR PARTICULATE CLEANLINESS (LPC test)

The main challenge when working and manufacturing a product in a cleanroom is maintaining its cleanliness level according to the cleanroom classification.
The quality of the manufactured product is at stake! The issues can relate both to safety (to avoid any product failure or health risk) but also to the economic question (to avoid defects and therefore scrap).
Many industries have critical processes: microelectronics, semiconductors, nanotechnologies, space, aeronautics or precision optics, automotive painting, battery manufacturing, medical devices or pharmaceutical or health industry.
It is therefore necessary to limit the risk of contamination of the product by limiting the number of particles in the air and on surfaces.
The particulate cleanliness performance of nitrile or Latex cleanroom gloves must therefore meet both the requirements of the final product and the manufacturing process, according to the relevant standards.
To determine the number of particles, SHIELD Scientific tests its ultra-clean cleanroom gloves using the LPC test method (Liquid Particle Count= Particulate test in liquid phase) according to the test method recommended in IEST-RP-CC005.04. For the purposes of testing the chemical composition of the gloves and in particular extractable ions, SHIELD Scientific uses the ion chromatography (IC) method.
WHAT DOES CLEANROOM PARTICULATE CLEANLINESS MEAN?
Dust or dirt on an optical component, wafer, microchip, implantable medical device or in an injectable solution can lead to devastating consequences.
Cleanroom concept, also called Controlled Atmosphere Zone (ZAC), is therefore designed to protect the manufacturing processes which guarantees a level of cleanliness by controlling potential sources of alteration (contaminant).
Not all critical environments have the same requirements or the same expectations in terms of cleanliness: some will mainly seek to control biological contaminants or dust conveying biological agents (pharmaceutical industry), while others will want to eliminate any microscopic particles (ionic extractable elements such as Zinc or Silicone for example in high-tech industries).
The major sources of cleanroom contamination come from staff, equipment, and materials.
When employees are moving or performing a task, they disperse thousands of particles, potentially carrying microorganisms, which can settle anywhere.
Cleanroom garments worn as personnel protective clothing are also a potential source of contamination. Indeed, they can release fibres but also particles into the air and on the surfaces. It is therefore essential to select personal protective equipment that is both compliant with:
- The cleanroom classification according to ISO 14644: classification of cleanrooms from ISO 1 to ISO 9 according to the maximum accepted concentration of particles per cubic meter of air, for particles from 0.1 μm to 5 μm.
- But also, for the pharmaceutical industry, with Good Manufacturing Practices (GMP) Annex 1: classification of cleanrooms according to a scale from A to D (A being the highest level of cleanliness) by distinguishing the maximum number of particles per cubic meter of air (Size particle equal to or greater than 0.5 μm and 5 μm) at rest and in activity.
Given that single-use nitrile or Latex gloves are a potential source of contaminants, it is important to assess their level of particles and ionic extractables to select ultra-clean cleanroom gloves suitable for the application.
WHAT IS THE CLEANROOM GLOVES LPC TEST (LIQUID PARTICULATE COUNT)?
The LPC test method is detailed in the IEST-RP-CC005.4 which is a “Recommended Practice” by IEST (Institute of Environmental Sciences and Technology – USA). It relates to “Gloves and fingers used in cleanrooms and other controlled environments”. It applies particularly to barrier gloves, to limit contamination.
IEST-RP-CC005.4 describes procedures and methods for measuring cleanroom glove cleanliness as well as their physical and chemical properties. The data obtained will help users select the appropriate glove for their application taking into account the requirements of their process and their ultra-clean environment.
The LPC test measures the size and distribution of particles in a liquid or on solid samples.
This test is performed in a cleanroom or controlled atmosphere workstation that meets the requirements for air cleanliness of ISO class 5 or higher.
The LPC test for single-use cleanroom glove consists of:
- Taking a glove with stainless steel tongs.
- Immersing the glove in a decontaminated glass beaker containing 750 ml of deionized water (very clean water normally containing no ion).
- Placing the beaker on a laboratory orbital shaker and shake at 150 rpm for 10 minutes before removing the glove from the beaker (this is to try to “release” the particles from the glove).
- Determining the number of particles present in the beaker according to their size using a particle counter.
- Analysing the particles to find out which element of the periodic table it is (extractables).
Figure 1 – Cleanroom gloves LPC test description
That’s how the level of particles and the levels and types of elements released by the glove are determined and therefore, the level of cleanliness of the cleanroom glove.
The specifications (thresholds not to be exceeded – Attention: not to be confused with the nominal values) of each glove are normally set out in a data sheet available to users, online or in paper or electronic form. SHIELD Scientific provides specific batch traceability of its nitrile and Latex cleanroom gloves. Batch data is reported in a “Certificate of Conformity (CoC)” including:
- The name and description of the glove, item codes, lot number and date of manufacture.
- Physical properties tests of the glove.
- The results of particulate counting tests performed according to IEST-RP-CC005.4.
- A counting of listed ionic contaminants also carried out in accordance with Method IEST-RP-CC005.4. An endotoxin record for sterile gloves.
WHY SELECT SHIELD SCIENTIFIC ULTRA-CLEAN GLOVES?
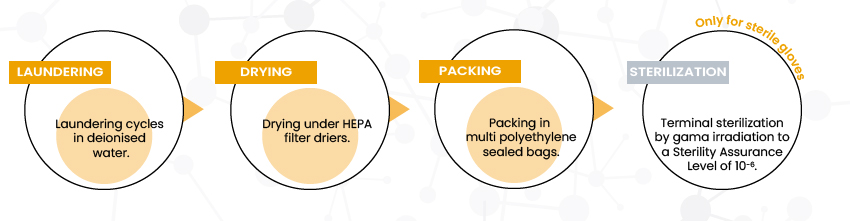
To achieve low particle and ionic extractable levels, gloves intended for cleanroom activities follow a very strict washing process after the so-called “online” dipping manufacturing phase, this is the “offline” process (after glove stripping):
Figure 2 – SHIELD Scientific Cleanroom gloves decontamination steps
For all SHIELD Scientific cleanroom gloves, these critical decontamination steps are performed in controlled atmosphere areas (cleanrooms).
The level of cleanliness of ultra-clean gloves differs mainly according to the number of wash cycles in deionized water to which they are subjected.
To facilitate the selection of cleanroom nitrile and Latex gloves according to the user’s application, we have therefore chosen to differentiate our single-use nitrile and Latex cleanroom gloves as follows:
Figure 3 – SHIELD Scientific’s cleanroom gloves differentiation
Our glove selection guide will make it easy to select the right glove for your application.
Note that the more a glove is washed and therefore ultra-clean, the more likely it is to be slippery. That’s why SHIELD Scientific offers, especially to the microelectronics industry, its ultra-clean SHIELDskin XTREME™ White Nitrile 300 DI++ glove with textured fingertips and a specific surface treatment for better grip.
In a cleanroom, contamination control is crucial to ensure compliance with the process and product quality. It is therefore of the utmost importance to select ultra-clean cleanroom gloves with cleanliness performance in accordance with the characteristics and requirements of your critical applications.
ASK NOW FOR SAMPLES OF OUR CLEANROOM GLOVES FOR TESTING AND VALIDATION BEFORE USE!
Share this interesting information