CONTROLLING ENDOTOXIN CONTAMINATION ON STERILE CLEANROOM GLOVES

Injectable pharmaceuticals or implantable medical devices contaminated with endotoxins can cause a very serious patient reaction.
Endotoxin contamination of batches of injectable solutions or implants can also have significant financial consequences for the manufacturer.
Therefore, monitoring for endotoxin contamination on sterile cleanroom nitrile gloves and latex gloves is a key concern for critical environments, including the implantable medical devices industry and the pharmaceutical industry.
What are bacterial endotoxins and their impact?
What are bacterial endotoxins?
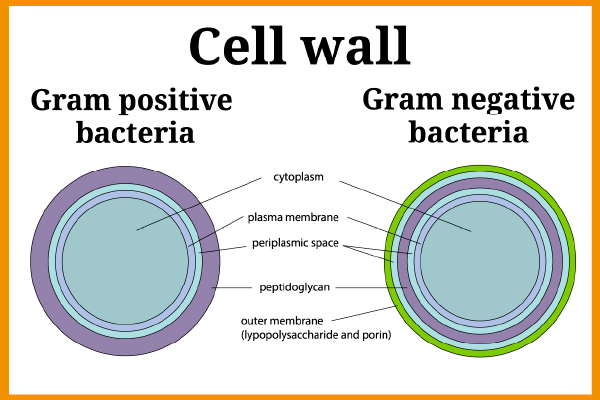
Endotoxins or Lipopolysaccharides (LPS) are fragments from the outer membrane of Gram-negative bacteria (using the Gram staining technique, Gram-negative bacteria produce a pink stain whilst it is purple for Gram-positive bacteria). Some examples of Gram-negative bacteria are E. coli, Salmonella, Legionella, Neisseria etc.
Figure 1 – Cell wall of Gram positive and Gram negative Bacteria
Gram-negative bacteria exist everywhere in the environment (in water, heating systems, digestive tract, and feces …) and therefore the presence of endotoxins may pose a very high risk. Endotoxin molecules are released during lysis (destruction of the membrane of a cell under the action of a physical, chemical, or biological agents).
What are the consequences of endotoxin contamination?
The biological makeup of endotoxins impacts the human and animal immune system and can cause an allergic reaction. Consequently, they can lead to relatively strong inflammatory reactions. They can also lead to serious infections in weakened (immunocompromised), young (preterm infants) or elderly patients.
The most common reaction is fever. This is why Gram-negative endotoxins are considered to be “pyrogens”, i.e., molecules that trigger a rise in body temperature by activating the immune system.
Endotoxins, when inhaled, may also cause Acute Respiratory Distress Syndrome (ARDS). On entering the body after an injection, endotoxins can cause disseminated intravascular coagulation or even septic shock when a large number reaches the bloodstream.
In critical environments such as the case in the pharmaceutical industry, endotoxin monitoring is essential. This is especially true in the production of pharmaceutical grade water (purified water), plus parenteral and injectable drugs manufacturing (infusion fluids, dialysis fluids…) and implantable medical devices.
Reducing endotoxin levels on cleanroom gloves
Endotoxins are known to be the most common source of pyrogens in pharmaceuticals and medical devices.
Endotoxins are membrane residues of Gram-negative bacteria. The latter are viable and numerous in the environment. In addition, endotoxins are highly resistant to disinfection or sterilization processes including gamma-irradiation.
This is why the bioburden of nitrile and latex gloves should be limited as much as possible before sterilization.
Bioburden is the concentration of all kinds of viable microorganisms on a surface or in a device. This bioburden is mainly from:
- Raw materials used during the production process (including pharmaceutical grade water).
- Operators involved in the manufacturing process.
- Cleaning and packaging operations of manufactured products.
The detection and quantification of microbial contamination on SHIELD Scientific sterile gloves is undertaken by an external organization and in accordance with ISO 11737-1. It is a fundamental part of the SHIELD Scientific’s quality control process.
Thus, all raw materials, packaging or protective equipment used in the manufacture of pharmaceuticals or implantable medical devices must comply with the health requirements for not only sterility but also pyrogenicity.
Sterility: To ensure a Sterility Assurance Level (SAL) of 10-6 (in accordance with the requirements of the European Pharmacopoeia) gloves must undergo microbiological validation in accordance with ISO 11137-2:2015 (Sterilization of health care products – Radiation – Part 2: Establishing the sterilization dose).
Figure 2 – SHIELDskin XTREME™ sterile gloves PE bag with sterilization indicator
Apyrogenicity: Depyrogenation can be done in many ways:
- Washing with Water for Injections (WFI) or other high-grade water such as deionized water.
- Dry heat treatment by exposure to high temperatures.
- Moist heat treatment by combination of pressure and hydrogen peroxide (Warning: an autoclave does not destroy endotoxins).
- Ultra filtration of endotoxins according to their molecular weight.
- Destruction by ethylene oxide.
Why is it important to control the endotoxin levels on sterile cleanroom gloves?
The sterility requirement (no living microorganisms present) and pyrogenicity (absence of pyrogenic molecules such as endotoxins) are paramount for many manufacturers of pharmaceuticals and implantable medical devices.
To limit, as much as possible, the risks associated with endotoxins, contamination should therefore be controlled throughout the production processes, from raw materials to finished products, including the protective equipment of the cleanroom operators.
Indeed, the gloved hand can be in direct contact with injectable solutions or their containers and implantable medical devices. Gloves are therefore potentially a source of contamination.
Figure 3 – Filling machine needle changed by an operator wearing double pair of SHIELD Scientific sterile gloves
Standard nitrile and latex single-use gloves undergo minimal treatment once they have completed the dipping and drying steps. The gloves’ cleanliness level may not therefore be acceptable for cleanroom applications. Also of note is that gloves can be heavily loaded with Gram-negative bacteria. Even sterile cleanroom gloves, which will typically be chlorinated and washed in purified water, often have no guarantee in terms of endotoxin levels.
In addition, since particles can transmit endotoxins, selecting gloves with a “guaranteed low level” of particles could help to strengthen your contamination control strategy.
Therefore, selecting gloves that have a low endotoxin claim (<20 EU/pair as defined in EN 455-3:2015) and, even better, that are batch tested for endotoxins may be a solution.
How is the endotoxin level of sterile cleanroom gloves monitored?
Regulatory Framework related to endotoxin contamination of sterile gloves
Annex 1 of the European Union’s GMP is one of the main documents governing the manufacture and control of medicinal products. This document provides guidelines for Quality Risk Management. In particular, the new GMPs’ Annex I specifies that microbial, particulate and endotoxin/pyrogen contamination of final products must be avoided. Sterile cleanroom gloves must therefore be integrated into the Quality Risk Management (QRM).
The EN 455-3:2015 (Single-use medical gloves – Part 3: Requirements and tests for biological evaluation) requires manufacturers of sterile single-use gloves to control endotoxin contamination of gloves. The test methods shall comply with the requirements of the European Pharmacopoeia, Monograph 2.6.14.
The horseshoe crab to detect endotoxins
The most widely used and recognized method for measuring the level of bacterial endotoxins on sterile cleanroom gloves is the one using Limulus Amebocyte Lysate (LAL).
The horseshoe crab is an ancestral marine arthropod that has the peculiarity of having blue blood (or rather a hemolymph in the case of an invertebrate).
Figure 4 – Horseshoe crab-Limulus polyphemus
This hemolymph contains amebocyte cells with an ovoid shape, which lose this shape and aggregate into a clot in the presence of endotoxins. This coagulation of Horseshoe crab blood is the basis for the various endotoxin determination test methods.
Test methods for determination of endotoxin contamination of sterile gloves
The outer surface of a pair of gloves is first subjected to an extraction of 40 to 50 ml of endotoxin-free water (LAL water in accordance with the European Pharmacopoeia) for 40 to 60 minutes at a temperature between 37 ° C and 40 ° C. The extract is then, if necessary, centrifuged (2,000 g for 15 minutes) to remove the particles. It is then decanted before being immediately subjected to an endotoxin test.
There are 3 main bacterial endotoxin test methods using Limulus Amebocyte Lysate:
- Gel-clot test (A “qualitative” test = can detect the presence of endotoxins but without being able to precisely determine the quantity): formation of a gel (“clot”) after mixing the extract and the LAL agent.
- Kinetic turbidimetry test (A “quantitative” test = allows to detect the presence of endotoxins and determine the quantity): development of turbidity after mixing with the LAL reagent.
- Chromogenic test (A “quantitative” test = allows to detect the presence of endotoxins and determine the quantity): development of a color after mixing with the LAL reagent.
The test result is reported in Endotoxin Unit (EU) per pair of gloves.
EN 455-3:2015 states that in order to be labelled “low endotoxin content”, gloves must have an endotoxin content limit of 20 EU per pair of gloves.
SHIELD Scientific low endotoxin content sterile gloves for critical environments
To limit the risk of some complications in patients, care should be taken to use good quality sterile gloves with a low endotoxin content.
As part of an overall contamination control and Quality Risk Management (QRM) strategy, it could be helpful to think about glove selection and how this applies to your critical environment.
That’s why SHIELD Scientific has developed a range of nitrile and latex cleanroom gloves that offer different levels of cleanliness directly related to the amount of washing in deionized water. For this range of single-use gloves, there is a simple classification:
DI: Gloves single washed in deionized water for basic contamination control and a particle level specification < 3,000 per cm² > 0.5μm.
DI+: Gloves triple washed in deionized water for high contamination control and a particle level specification < 1,200 per cm² > 0.5μm.
DI++: Gloves multi washed in deionized water for extreme contamination control and a particle level specification < 850 per cm² > 0.5μm.
All SHIELDskin XTREME™ brand sterile gloves are designed to meet the quality standards required by operators working to GMP in order to reduce the risk of contamination. All SHIELD Scientific cleanroom nitrile and latex sterile gloves are batch tested to demonstrate a low endotoxin content of < 20 UE (Endotoxin Unit) per pair of gloves and a Sterility Assurance Level (SAL) of 10-6 (by gamma irradiation sterilization).
A Certificate of Irradiation (CoI) is provided with each batch of SHIELD Scientific sterile nitrile and latex cleanroom gloves. A Certificate of Conformance (CoC) provides batch-specific data on levels of particles and endotoxin.
BECAUSE CONTROLLING ENDOTOXIN CONTAMINATION IS VITAL IN CRITICAL ENVIRONMENTS, DON’T WAIT ANY LONGER TO ORDER SAMPLES OR ASK ADVICE FROM A SHIELD Scientific SALES REPRESENTATIVE.
Share this interesting information